Preventing Cardiovascular Diseases with an AI-Powered Rapid Diagnostic Test
Keerthi Kodithuwakku is a biomedical engineer, the co-founder and CEO of Jendo Innovations, a bio-medical startup delivering patented healthcare solutions detecting abnormalities in the cardiovascular system and seeking to prevent the risk of cardiovascular diseases.
His strong passion for biomedical engineering resisted adverse advice from some senior advisors in the healthcare industry about the lack of prospects in biomedical engineering in Sri Lanka. Along with a like-minded group of engineers, he followed his dream of providing a solution to help solve medical conditions. In their research and entrepreneurial journey, Keerthi said that he found strength and motivation through the strong support of his wife, family, friends, and coworkers in overcoming the obstacles faced when developing Jendo Innovations.

“We went through a lot of medical problems and we identified a new technology to detect endothelial dysfunction which is an early detection of cardiovascular diseases,” he recalled. The endothelium describes the deepest cell layers of blood vessels. It was just a concept at that time, Keerthi said, “but going through the literature, we realized cardiovascular diseases (CVDs) are a huge health issue, and the technology would apply to all people in the world.” “We were inspired by that, and that was the original idea of Jendo.” According to the World Health Organization, (CVDs) are the leading cause of death globally and claim millions of lives a year.
AI in cardiology – A Steppingstone for Jendo Innovations
The key developers of Jendo innovations: Keerthi Kodithuwakku, Isuru Rajakaruna, and Charith Vithanage found the most amount of inspiration towards the end of their tenure in undergraduate studies at the University of Moratuwa. This is when they started working on an artificial intelligence-based device identifying abnormalities in the cardiovascular system to predict the risk of disease.
Straight out of university, in 2015, Keerthi co-founded Jendo Innovations with his co-inventors along with Heminda Jayaweera, and Vinod Samarawikrama to commercialize the invention. Keerthi, now 32, said “another motive behind the formation of a registered company was to create a platform and provide opportunities for upcoming biomedical engineers in Sri Lanka to be a part of the Jendo Innovations journey and make good use of their intellectual resources and improve the overall standard of biomedical engineering in the country.”
Jendo comes from the two first syllables of “endothelium,” and refers to Indonesian martial art “since it is a fight,” he explained.
Endothelial Dysfunction, Disease Tattling
Cardiovascular diseases are silent killers, as they often go unnoticed for 20 to 30 years. The progressive worsening of the patient’s conditions goes through multiple stages, from inflammation to the formation of plaque, to the stiffening of walls, ultimately leading to life-threatening blood clots.

worsening with the years
Endothelial dysfunction is the first detectable stage in that process, explained Keerthi. Jendo is a method for monitoring the vascular system’s health by detecting pulse abnormalities. Endothelial dysfunction is also involved in numerous systemic diseases such as cerebrovascular disease, such as strokes, metabolic syndrome, erectile dysfunction, renal failure, claudication, sleep apnea, gangrene, and pre-eclampsia toxemia, he said.
A Fifteen-Minute Heart Check-Up that Can Save Lives
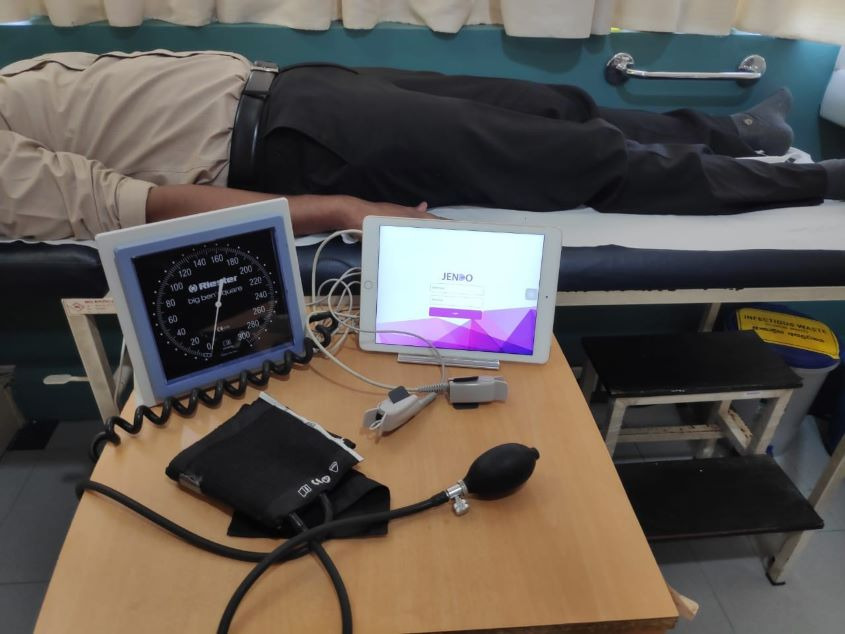
The entire Jendo procedure takes about 15 minutes. “We are not only detecting the blood flow but we create artificial conditions by stopping the blood flow for five minutes in the right thumb of the patient, fitted with a red and infrared light-emitting sensor, then releasing it,” Keerthi detailed. “By doing that the endothelium starts reacting to that artificial condition.”
Complex algorithms in the system are used to detect endothelial activity and blood flow of a patient’s circulatory system. The pulse and temperature signals extracted from a patient are used to create a machine-learning model that will be used to perform analysis. Upon evaluation of the condition of the patient’s blood vessels, the system provides a prediction of the cardiovascular risk level. Finally, a report is generated with the results, along with some lifestyle recommendations.
“We recommend that people take the test in a medical facility under the guidance of a nurse,” Keerthi said, adding that people could take the test at home providing they closely follow the procedure.
Tried and Tested on Patients to Prevent Heart Disease
Jendo’s system is not commercialized yet but the device is already being used by hospitals and laboratory chains, and clinically tested on over 800 patients.
Under the current business model, the device will be sold at US$2,250 with an annual subscription fee of US$10,000. The test will be charged US$8. According to Keerthi, “the screening price is affordable in comparison with other established procedures.”
Patent Key to Gain Investors’ Trust in Jendo
The company swiftly proceeded to apply for a patent, first in Sri Lanka (pending), then in the United States (granted: USPTO Patent 10,912,464 B2), and in Japan (Pending), through the WIPO Patent Cooperation Treaty (PCT).
According to Keerthi, investors want to make sure the products they invest in is ahead of the competition. “A patent is key to proving the value of your invention and winning investors’ confidence,” he said.
Healthcare Regulatory Compliance Challenges for Medical Technology
One of the challenges that the company is facing is finding partners in the health ecosystem. The other one is going through the health regulatory process as each country has its regulatory authority. Sri Lanka’s regulatory authority is currently reviewing the Jendo system. Once the review is completed, it will allow the commercialization of the device. The company will also apply for the United States Food and Drug Administration (FDA) approval, followed by other countries.
Jendo Innovations is also in discussion to establish two business centers outside of Sri Lanka; one in Switzerland, and the other one in Singapore. “Both countries have a favorable business environment, and growth opportunities,” according to Keerthi.

Mind-Controlled Drone and AI for Diabetic Retinopathy Screening
In addition to Jendo Innovations, Keerthi has launched several other startups. Under these companies (Effective Solutions, Optha Innovations), other innovative medical devices and systems are being developed.
One of them is Myndrone, which allows the user to control a drone by using his/her brain waves. “This activity relaxes the brain and the user feels calm after a game session”, Keerthi said.

Another product soon to be launched is Ophta-AES, a Human Machine Interface (HMI), an AI-powered system to provide early detection of diabetic retinopathy, which is the most common cause of blindness among diabetic patients worldwide.
“Over 45 percent of diabetic patients worldwide develop diabetic retinopathy and most of them could prevent worsening of the disease with early detection”, Keerthi explained



