By Anatole Krattiger, Global Challenges Division, WIPO
Over the last three decades, medical technologies have transformed many previously untreatable diseases – such as HIV/AIDS – into manageable long-term conditions. However, as the global disease burden evolves there is a continuing need to develop new and more effective medicines. The challenge for policymakers is to establish an environment that stimulates health innovation while ensuring widespread access to new, more effective products to address unmet global health needs.
The issues of innovation and access are inevitably intertwined, cutting across distinct policy areas, in particular, public health, intellectual property (IP) and international trade (see figure 1). Finding the right balance between health, trade and IP policies to sustain innovation and ensure widespread access to life-saving technologies is one of the primary public policy challenges of our time.
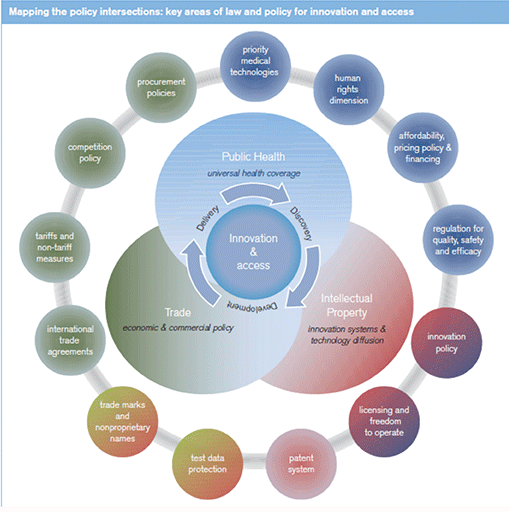
Intersection between public health, intellectual property and trade.
A recent study by the World Health Organization (WHO), WIPO and the World Trade Organization (WTO) entitled Promoting Access to Medical Technologies and Innovation – Intersections between public health, intellectual property and trade  , seeks to improve understanding of the options available to policymakers in developing effective public health strategies that address the growing demand for innovation and access. Several elements of the IP system are relevant to this debate, in particular, patents, and the protection of test data.
, seeks to improve understanding of the options available to policymakers in developing effective public health strategies that address the growing demand for innovation and access. Several elements of the IP system are relevant to this debate, in particular, patents, and the protection of test data.
The role of medical technology
Technology is unquestionably an essential component of public health. Medicines such as antibiotics and antiretrovirals have dramatically improved health outcomes in the same way that technologies such as medical imaging have transformed diagnosis and treatment. Developing these complex products is an expensive and risky business. Unlike other areas of technological development, medical innovation is all the more challenging because of the ethical dimension of medical research, rigorous regulatory oversight, liability issues, high costs and high failure rate. This helps explain why IP protection is so important for companies involved in medical research and development (R&D).
The rationale of the IP system
The rationale of the intellectual property (IP) system in general, and the patent system in particular, is to make investment in innovation attractive and to offer a mechanism which ensures that the knowledge contained in patent applications is accessible to society. In this way, it seeks to balance competing private and public interests.
Anyone applying for a patent is required to disclose the details of their technology so that the public is aware of, and can eventually use, the knowledge contained in patent documents. Patent information available through public databases, such as WIPO′s PATENTSCOPE, offers useful insights about innovation trends and freedom-to-operate, and can help shape patenting and licensing strategies. Data indicate overall long-term growth in patenting of medical technologies (a sign of renewed investment in this area) and that an increasingly diverse range of public and private users (see Figures 2 and 3), including from emerging economies, are using the international patent system.
While the patent system is designed to promote innovation by providing an incentive to invest in R&D, the impact of patents on access to medical technologies is complex and much debated. Just as the existence of a patent need not be a barrier to access, the absence of a patent right does not guarantee effective access. As noted in the WHO′s Framework for Access to Medicines, access to medicines is rarely dependent on a single factor; it also includes rational selection and use of medicines, affordable prices, sustainable financing and reliable health and supply systems, among others.
Striking an appropriate balance
Striking an appropriate balance between encouraging medical innovation and enabling access to it has been a major preoccupation of policymakers, health activists and the private sector, since the 1990s when concerns about access came to the fore in relation to the treatment of HIV/AIDS in many African countries. The WTO′s Doha Declaration on the TRIPs Agreement and Public Health of 2001, clarified a number of rules specific to IP and helped reassure the global community that IP should not prevent access to the medicines needed in developing countries.
Medical technologies are usually very expensive to develop but relatively cheap to reproduce. Without the protection conferred by a patent it would not be financially viable for companies to continue investing in research, product development and regulatory approval. If competitors could “free ride” on the cost of developing a product and were able to immediately introduce their own versions, the inventor would not get the expected financial returns thereby weakening any incentive to develop new products.
The search for new medical innovation strategies
In recent years, the rising cost of medical research has not been matched by a proportionate increase in new products entering the market. This has sparked a rich debate about how to improve innovation models and strategies and how to finance medical R&D to address unmet global health needs.
A variety of “push” and “pull” mechanisms are under discussion. “Push” mechanisms encourage medical research when the outcome is not clear, and may include grant funding and tax credits. They are particularly useful in building-up knowledge relating to neglected tropical diseases. “Pull” mechanisms include prizes, and Advance Market Commitments and Advanced Purchase Commitments which offer certain guarantees to encourage companies to develop solutions for diseases where no sustainable market exists.
In most developed countries, social insurance provides an infrastructure that enables patients to have access to healthcare technologies while also ensuring that those responsible for developing new medical products are paid for their innovations. In many developing and least developed countries (LDCs), however, social insurance systems are less comprehensive and many patients do not have access to the life-saving interventions they need.
An evolving medical research landscape
Market-based innovation models have largely failed to address the neglected tropical diseases specific to developing countries. The identification of this gap in research has prompted significant developments in the medical research landscape.
Multi-sectoral public-private partnerships, for example, such as those developed to tackle the HIV/AIDS crisis, are proving instrumental in developing effective health products and policy solutions.
Product development partnerships usually involving non-profit organizations, foundations and industry are helping to identify and overcome bottlenecks to research into neglected tropical diseases and have significantly increased the number of products in development for these diseases.
Other partnership models are also emerging. The WIPO Re:Search initiative launched in October 2011, is designed to accelerate the development of drugs, vaccines and diagnostic tools to treat neglected tropical diseases, malaria and tuberculosis. The consortium, which now has over 70 members, brings together the private and public sector research communities to develop research partnerships and provide access to IP, on preferential terms, for pharmaceutical compounds, technologies, patents and most importantly, know-how and data for researchers working on these diseases. (See Catalyzing research into neglected tropical diseases).
Creative licensing strategies
Creative licensing strategies, such as patent pools, are also proving helpful in building the partnerships required to accelerate medical innovation. A patent pool is a consortium of at least two companies that agree to cross-license patents relating to a particular technology on fair, reasonable and non-discriminatory terms. Within the health field the Medicines Patent Pool Foundation aggregates antiretroviral drug patent rights and licenses them to generic drugs manufacturers. Similarly, MPEG LA′s Librassay® service acts as a licensing “supermarket” for diagnostic and research tool patent rights in support of molecular diagnostic testing for the development of personalized medical therapies.

Among the top ten countries of origin are the United States, Japan, the Republic of Korea and a number of
Western European countries
The IP operating context
The multilateral legal IP framework, as defined by various WIPO-administered treaties and the Agreement on Trade-Related Aspects of Intellectual Property (the TRIPS Agreement) administered by the WTO, provides the context and general guiding principles for the operation of national IP systems.
The TRIPs Agreement, which incorporates substantive provisions of several WIPO-administered treaties, has significant implications for the application of IP to medical technologies. In particular, it requires that patents are available for inventions in all fields of technology, provided they are new, involve an inventive step (or are non-obvious) and are capable of industrial application (or useful). It also seeks to balance the rights and obligations of producers and users of technological innovation when it comes to the protection and enforcement of IP rights.
The TRIPS Agreement also requires the protection of clinical trial data against unfair commercial use although it leaves countries great scope in determining how to do so. This area further illustrates the complex relationship between IP, innovation and access and the dilemmas facing policymakers.
To obtain authorization to market new medicines, companies need to undertake pharmacological and toxicological tests and clinical trials to demonstrate safety and efficacy. In light of the considerable time and money required to produce these data they qualify for protection under the IP system. There is, however, often strong competing public interest in securing early access to these data for the manufacture of generics.
Balancing competing interests
A wide range of policy options and flexibilities have been built into the IP regime to accommodate diverging national public health interests and objectives. Empirical evidence suggests, however, that a better understanding of how to implement these flexibilities is required to ensure that national IP regimes respond to the individual needs and policy objectives of each country.
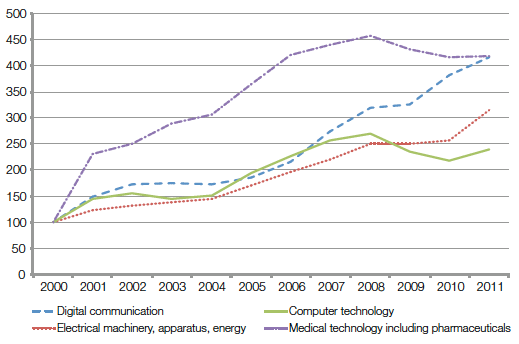
The term “medical technologies” as used in the study includes data relating to medical technology (6.6 percent
of all PCT filings in 2011) and pharmaceuticals (4.7 percent of all PCT filings in 2011). In this consolidated
form (11.3 percent of all PCT filings in 2011), medical technologies, including pharmaceuticals, represent the
field of technology with the highest number of PCT filings between 1978 and 2011.
Key flexibilities in the field of patent law to improve access to medicines for both communicable and non-communicable diseases, include:
- transition periods for LDCs;
- choice of patent right exhaustion regimes - such regimes limit the extent to which patent holders can control a patented product after authorized sale;
- refining the criteria for grant of a patent;
- opposition procedures;
- exceptions and limitations to patent rights, including the regulatory review (“Bolar”) exception to facilitate market entry of generics;
- compulsory licenses and government use authorization where the responsible authority grants specific permission to a person other than the patent owner to produce, import, sell or use a patent-protected product or process for a specific requirement.
International trade and access
International trade is critical to enabling access to medicines, particularly for smaller countries with no domestic manufacturing capacity. Trade stimulates competition and improves economies of scale, which in turn reduce prices and spawn a wider range of suppliers, improving stability of supply. Trade policy also has an important bearing on efforts to build domestic production capacity in medical products and can directly affect accessibility to pharmaceutical ingredients and medical technologies.
The policy and legal framework for international trade has become more complex with the proliferation of bilateral and regional free trade agreements. The overall impact of these agreements on access to medicines, however, is yet to be systematically analyzed. Such analysis is necessary to ensure that future agreements retain an appropriate balance between innovation and access.
While there are no simple solutions to the complex challenge of spurring medical innovation while securing access, the trilateral study sheds light on the complex interplay between health, IP and trade policies and offers a sound basis for future policy debate and analysis.

